Science hasn’t shown these medications work. They’re being sold anyway
Federal regulators are increasingly approving medicines before studies have shown they work, leaving patients at risk of taking prescriptions that could harm but not help them.
Last year, 14 new drugs received so-called accelerated approval, in which they have not gone through the testing that the Food and Drug Administration regularly requires. That amounted to 28% of the 50 drugs the FDA approved. The numbers have jumped from 2018 when just four, or 7%, of the 59 new drugs were approved under those rules.
The rules were created to be used in rare cases in which seriously ill patients had no other treatments. But with pressure from the pharmaceutical industry, patient groups and politicians to speed medicines to market, now most drugs are approved under the accelerated approval rules or through three similar programs requiring less evidence and regulatory scrutiny.
The shift has alarmed some experts who worry the industry is exploiting the rules to sell medicines of questionable effectiveness and safety at sky-high prices.
“This is causing huge amounts of real harm,” said Jerome Hoffman, UCLA professor emeritus of medicine, pointing to side effects and medical bills patients simply can’t afford.
Although the FDA has the power to remove these drugs when studies later show the medicines don’t work, that move has been rare.
A recent Times investigation detailed how Covis Pharma has refused the agency’s request to withdraw a drug for pregnant women at risk of premature birth. The FDA approved the drug called Makena a decade ago with hopes it would reduce deaths and serious disability among infants born too soon. The FDA required the company to perform a study to prove the drug’s benefits. That trial took eight years and “unequivocally failed to demonstrate” that Makena worked, agency scientists have said.
The drug Makena doesn’t reduce the risk of preterm birth, a study found, and the FDA recommended it be taken off the market. Its maker has refused.
Dozens of drugs now on the market haven’t yet been backed by studies confirming their effectiveness.
There is no requirement that patients be told they have been prescribed one of these drugs — a gap causing some doctors to fear that people are being misled.
“I think people generally assume that when the FDA has approved something, there’s overwhelming evidence it is safe and effective,” said Joseph Ross, a Yale professor of medicine and public health, who has written about how the rules should be reformed.
In a statement, the agency said it was “committed to ensuring the integrity of the accelerated approval program.”
“We believe patients who currently lack adequate treatment options for serious or life-threatening diseases are willing to accept some uncertainty regarding clinical benefit when a new treatment is developed,” the FDA said.
“In the vast majority of accelerated approvals,” it said, the drug’s clinical benefits were later verified through the confirmatory studies it required.
Pricey drugs not yet proven to work
The FDA is approving more drugs before studies show they work. Though their effectiveness hasn’t been proven, some hit the market with high list prices.
Exondys 51
Duchenne muscular dystrophy
$300,000 per year
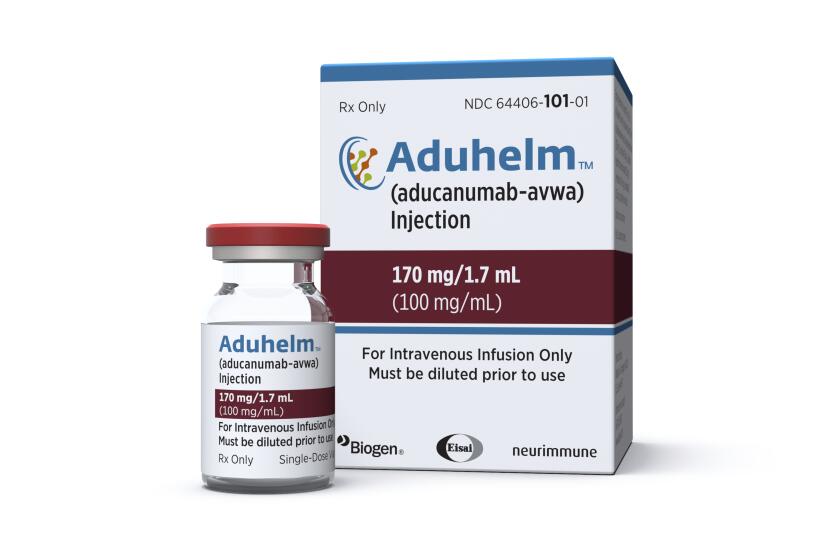
Aduhelm
Alzheimer’s disease
$28,200 per year
Makena
Preterm birth
$13,000 per pregnancy
For decades, the FDA’s approval standard had been two “adequate and well-controlled” studies showing “substantial evidence of effectiveness.”
In 1992, with the crisis of the AIDS epidemic, the agency started its accelerated approval program. The program allows companies to use what are called “surrogate endpoints” — certain signs in clinical trials that show a medicine might be beneficial for patients.
Many drugs approved under the program have been cancer drugs for which trials have not shown they lengthen lives. Instead the companies have used X-rays and other measures to show the drug appeared to cause a positive response.
Since 1992, Congress has passed laws adding more ways for drugs to get faster approval with less evidence than the FDA had long required.
The new programs were introduced to help patients with rare diseases and no other hope. Now they are commonly used.
Last year, 74% of the 50 new drugs approved by the FDA’s Center for Drug Evaluation and Research were granted some form of expedited approval. Among the medicines on that list are those for heart failure and lupus — conditions for which patients already have multiple medicines available.
“Forget two studies, forget well-done studies, forget randomized trials,” Hoffman said of the FDA’s increasing use of the expedited approvals. “It’s now if somebody says it might work and we haven’t yet proven it’s harmful — let’s try it.”
If a company is granted an accelerated approval, the FDA requires studies to confirm the drug works. But the agency has often not forced companies to complete these studies, which could halt sales if they fail.
“Companies drag their feet,” Ross said. “The studies don’t get done.”
Asked about the delayed trials, an agency spokesperson said, “The FDA will use every authority at our disposal to encourage the diligent initiation of well-designed confirmatory studies. If there are any gaps in the FDA’s ability to hold developers accountable for conducting studies as quickly as the science allows, then the agency will work with Congress to close those gaps.”
In a growing number of cases, companies continue to sell the drugs even after those studies are completed and show the drugs aren’t effective. Ross and other researchers call these “dangling approvals.”
“Makena is illustrative of this whole dance that’s taking place,” Ross said.
Covis has told the FDA it wants to keep selling Makena while it does more research to try again to show it’s effective. The company disputes the results of the study that failed to show it worked, saying it did not include enough Black women, who are at highest risk of preterm birth. Covis says the drug is safe and continuing prescriptions won’t harm pregnant women. Makena’s label lists side effects such as blood clots and hypertension. Some doctors worry about the risk of stillbirths. The evidence will be reviewed at a hearing the FDA has not yet scheduled even though it’s been three years since the trial showed Makena did not work.
Two recent accelerated approvals have raised more questions of whether the FDA has lowered the bar too far.
U.S. regulators have OKd the first new Alzheimer’s drug in nearly 20 years, against the advice of their own outside experts.
Last year, the agency stirred controversy when it overruled its committee of outside experts and granted approval to a drug called Aduhelm for Alzheimer’s disease, which affects 6 million Americans and has no cure. Three committee members quit in protest.
In the decision, the FDA used evidence from clinical trials that showed the drug reduced levels of amyloid plaque in the brain. But scientists questioned the use of that surrogate marker, pointing out that other medicines have targeted the plaques with little effect on a patient’s dementia.
Infusions of the drug can cause serious side effects, including swelling in the brain.
Biogen, the drug’s maker, introduced Aduhelm at a price of $56,000 a year. The high price of the drug for a condition that affects millions of Americans caused federal officials to propose an increase to Medicare premiums of 14.5% to cover the billions of dollars the government expected to pay for it. In December, the company cut the annual price in half to $28,200.
In January, Medicare proposed that it would cover the cost of Aduhelm only for patients in clinical trials. A final decision is pending.
The FDA’s approval of an Alzheimer’s drug of dubious value will throw U.S. healthcare spending into crisis.
More concerns about the accelerated approval program were raised in 2016 when Janet Woodcock, a top FDA official, approved a drug for Duchenne muscular dystrophy despite conclusions of agency scientists that it did not work and its risks were not yet known.
Sarepta Therapeutics began selling the drug Exondys 51 at a price of $300,000 a year. The approval sparked a dispute inside the FDA that soon became public when documents were released.
“By allowing the marketing of an ineffective drug, essentially a scientifically elegant placebo, thousands of patients and their families would be given false hope in exchange for hardship and risk,” wrote Ellis Unger, one of the FDA scientists who decried Woodcock’s decision.
Woodcock defended her move. Among her arguments was that Sarepta “needed to be capitalized,” she told an internal board reviewing the dispute. She pointed out that the company’s stock went down when a committee of experts voted against the drug and then went up when she later sent a letter to Sarepta saying she expected to soon grant the drug accelerated approval.
She wrote that if the drug was not approved, Sarepta would have insufficient funding to continue to study Exondys 51 and the other similar drugs in its pipeline.
The agency spokesperson told The Times that “the FDA’s decision on any drug product, in any disease area, is based on an assessment of whether the benefits of the drug outweigh its risks. This assessment is informed by science, medicine, policy and judgment, in accordance with applicable legal and regulatory standards.”
Patients depend on doctors to keep them safe from medicines that have more risks than benefits. Yet one report suggests that most doctors don’t know about the scant evidence behind some drugs they prescribe.
In a 2016 survey, 70% of doctors wrongly believed that an FDA approval required studies showing both “a statistically significant” and “clinically important” effect.
“The drug company and all the advertising says this is a great new drug,” Hoffman said. “How many doctors are actually going to go and say, ‘Wait a second. Is that really true?’”
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.