There’s a new highly transmissible COVID-19 variant. Could FLiRT lead to a summer uptick?

Two new COVID-19 subvariants, collectively nicknamed FLiRT, are increasingly edging out the winter’s dominant strain ahead of a possible summer uptick in coronavirus infections.
The new FLiRT subvariants, officially known as KP.2 and KP.1.1, are believed to be roughly 20% more transmissible than their parent, JN.1, the winter’s dominant subvariant, said Dr. Peter Chin-Hong, an infectious-disease expert at UC San Francisco.
The two FLiRT subvariants combined comprised an estimated 35% of coronavirus infections nationally for the two-week period that began April 28, according to the U.S. Centers for Disease Control and Prevention. By contrast, JN.1 is now believed to comprise 16% of infections; in mid-winter, it was blamed for more than 80%.
“It’s been quite a while since we’ve had a new dominant variant in the U.S.,” said Dr. David Bronstein, an infectious diseases specialist at Kaiser Permanente Southern California. “With each of these variants that takes over from the one before it, we do see increased transmissibility — it’s easier to spread from person to person. So, that’s really the concern with FLiRT.”
The largest FLiRT subvariant, KP.2, is growing particularly fast as a proportion of coronavirus infections. In late March, it made up just 4% of estimated infections nationally; most recently, it’s estimated to make up 28.2%.
Americans want to forget about COVID-19, but fallout from the pandemic is shaping voter attitudes about the nation’s economy and divisive politics heading into a Biden-Trump rematch.
The new subvariants have been dubbed FLiRT for the mutations on the evolved COVID-19 virus. “So instead of an ‘L,’ there’s an ‘F.’ And instead of a ‘T,’ there’s an ‘R.’ And then they put an ‘i’ in to make it cute,” Chin-Hong said.
Despite their increased transmissibility, the new mutations don’t appear to result in more severe disease. And the vaccine is expected to continue working well, given the new subvariants are only slightly different from the winter version.
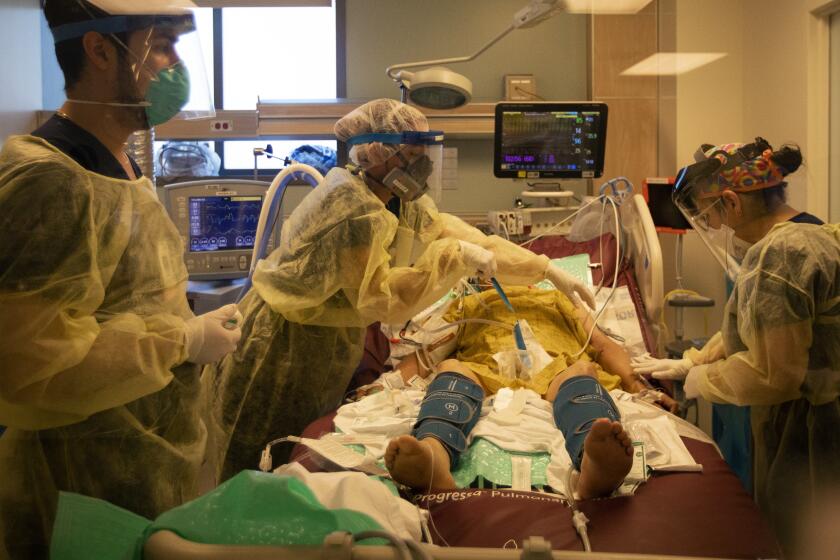
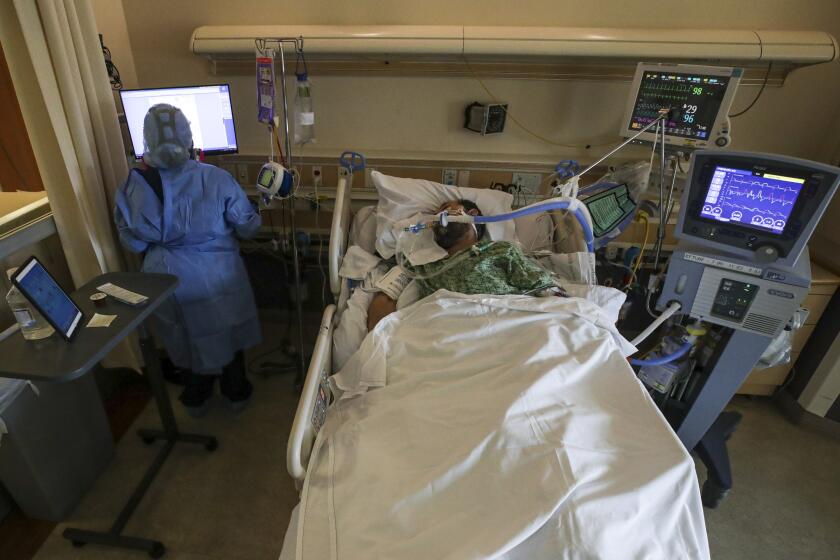
The entry of the subvariants also come as COVID-19 hospitalizations hit record lows. For the week ending April 27, there were 5,098 admissions — one-seventh of this winter’s peak, in which 35,137 admissions were reported for the week that ended Jan. 6.
However, as of May 1, hospitals nationwide are no longer required to report COVID-19 admissions to the U.S. Department of Health and Human Services; only voluntarily submitted data will now be posted nationally.
This past winter, the mortality rate for patients hospitalized with COVID-19 was 35% higher than for patients hospitalized with the flu.
In Los Angeles County, COVID-19 levels appear to be in a lull. For the week that ended April 27, coronavirus levels in L.A. County wastewater were at 8% of the winter peak.
Still, some doctors say they wouldn’t be surprised if there is a summer uptick in COVID cases — as has occurred in prior seasons.
“By the summer, we can expect people’s immunity to be a little bit lower,” Chin-Hong said. For those who are older or immunocompromised, “they are potentially at risk for getting more serious disease.”
Plus, people often gather indoors during summer to avoid the heat, which can increase the risk of transmission in crowded public venues.
The CDC and NOAA have launched a new HeatRisk indicator to help people avoid heat-related illness and protect themselves during high temperatures.
Chin-Hong said he is seeing COVID-19 patients at UC San Francisco with serious illness, and “they were either very old or very immune compromised and they didn’t get the most recent shots.”
That the FLiRT subvariants are more easily able to spread underscores how important it is for those most at risk to be up to date on vaccinations and stay away from those who are sick, doctors say.
And while the chance of long COVID is likely less than the early days of the pandemic, it still exists.
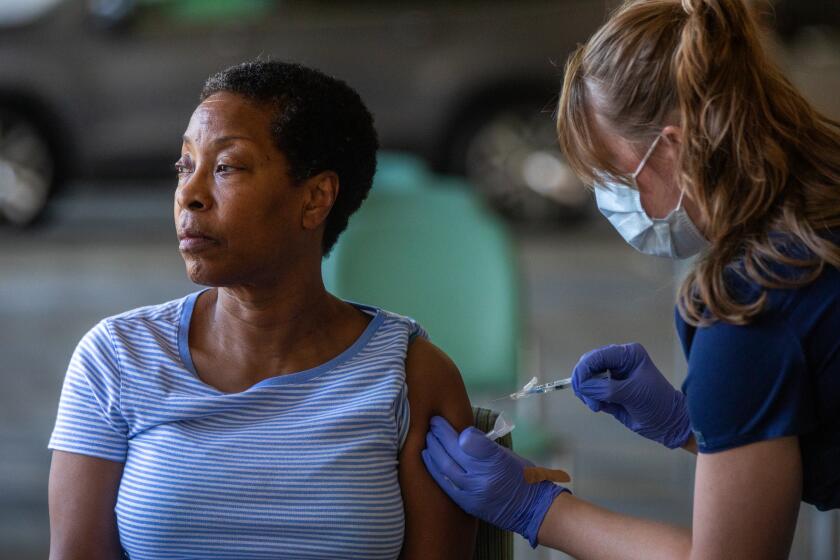
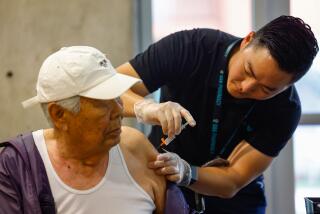
Many people haven’t gotten a recent COVID-19 vaccination, data show. For the week that ended Feb. 24, 29% of seniors nationwide had received a dose of the updated vaccine that became available in September. In California, as of April 30, about 36% of seniors had received an updated dose.
The U.S. Centers for Disease Control and Prevention recommend that seniors 65 and older get a second dose of the 2023-24 COVID-19 vaccination.
“We are still seeing those hospitalizations and bad outcomes, and even folks who are passing away from COVID. It hasn’t gone away,” Bronstein said. “The good news is that the ... vaccine still is very good at protecting you against hospitalizations, severe outcomes and death.”
Between October and April, more than 42,000 COVID-19 deaths were recorded nationally, according to the CDC. That’s significantly larger than the estimated flu deaths over the same time: 24,000.
Still, the number is smaller than the comparable period for the prior season, when more than 70,000 COVID deaths were reported. And that tally is far smaller than the first two devastating pandemic winters: Between October 2021 and April 2022, more than 272,000 deaths were recorded; and between October 2020 and April 2021, the number was more than 370,000.
The CDC in February recommended that seniors 65 and older get a second dose of the updated vaccine as long as it had been at least four months since an earlier injection. The CDC also says everyone 6 months and older should get a dose of the updated vaccine.
“Right now, the most important thing that folks can do is get the vaccine,” Bronstein said. He suggested those who are especially vulnerable continue to mask whenever possible, especially in places like crowded airports and planes.
In addition, he said, it’s important that people who are sick stay at home to avoid spreading germs to others, particularly the elderly. And if sickened people must leave home, they should wear a mask around others.
“Even in the summertime, what may feel like a cold can actually be a COVID infection,” Bronstein said. “We need to make sure that if you’re sick, that we’re testing whenever possible, staying home ... and make sure that your symptoms are more mild before you decide to go back to your regular activities.”
The CDC said people who test positive for COVID-19 no longer need to stay in isolation for five days and can return to regular activities if their symptoms are mild and improving and it’s been a day since they’ve had a fever.
California recommends that people with COVID-19 symptoms stay home until symptoms are mild and improving and they haven’t had a fever for 24 hours without medication.
They should also mask around others while indoors for 10 days after becoming sick or, if they have no symptoms, after testing positive. They can stop wearing a mask sooner, if they have two consecutive negative rapid test results at least a day apart. But they should avoid contact with all higher-risk people for 10 days, according to the state Department of Public Health.
And ahead of travel plans this summer, Chin-Hong suggested that older people speak with their healthcare provider about making sure that, should they come down with COVID-19, that Paxlovid can be prescribed without interfering with other medications. Paxlovid is an antiviral drug that, when taken by people at risk for severe COVID-19 who have mild-to-moderate illness, reduces the risk of hospitalization and death.
Chin-Hong also suggests that it makes sense for healthcare providers to prescribe Paxlovid to higher-risk people planning to travel where the medicine may not be readily available, as a “just-in-case” prescription. Clinicians have that discretion since Paxlovid has been fully approved by the U.S. Food and Drug Administration, which gives healthcare providers greater leeway in deciding when to prescribe the drug.
Earlier this year, another medicine was also made available to help protect the most vulnerable people — such as cancer patients and those who have received organ transplants. It’s a monoclonal antibody called Pemgarda, which is administered intravenously and can be given once every three months. Authorized by the FDA for emergency use, it’s given prophylactically and can help recipients prevent COVID-19 if they are later exposed to an infected person.
Anticipation is also building for a fresh version of the COVID-19 vaccine to be released possibly by September. It could be designed against last winter’s JN.1 strain, but it’s also possible officials decide it should be designed against the rising FLiRT subvariants, Chin-Hong said.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.