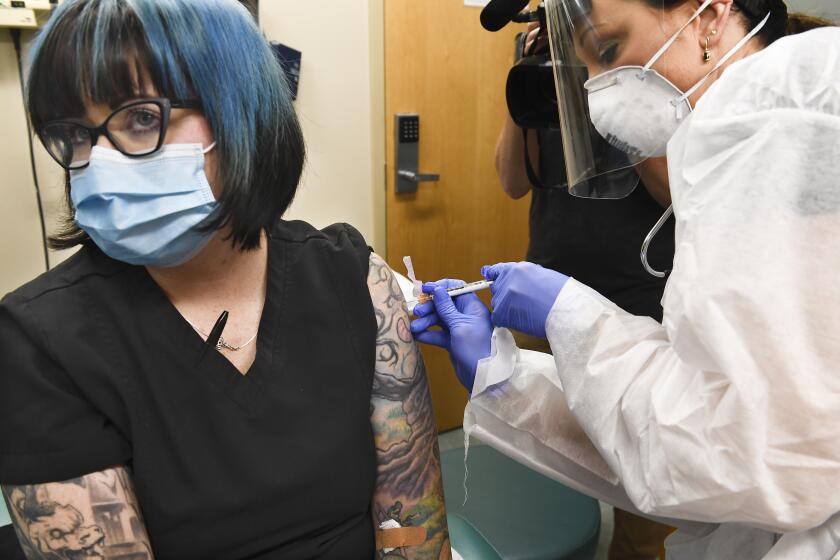
Data on Oxford and AstraZeneca’s COVID-19 vaccine expected by Christmas
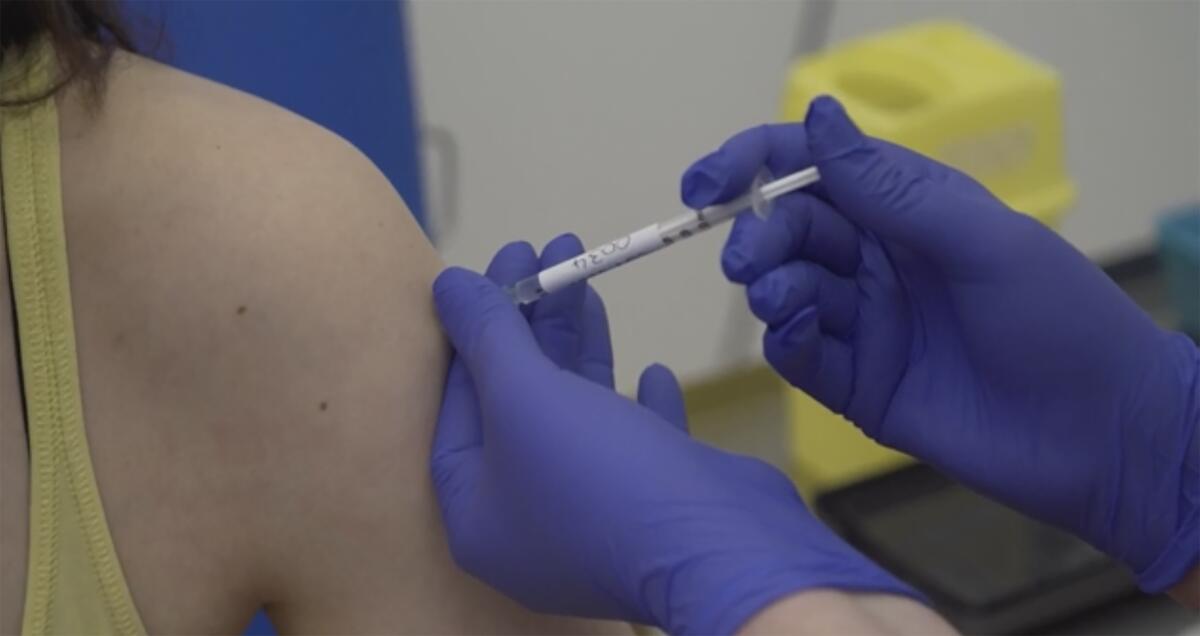
Oxford University scientists expect to report results from the late-stage trials of their COVID-19 vaccine by Christmas, a key researcher said Thursday, adding that early research has found the shot to be well-tolerated and to produce a strong immune response in people over 70.
The Phase III trials of the vaccine, which is being developed in conjunction with drugmaker AstraZeneca, are now generating the data needed to report results, said Dr. Andrew Pollard, an expert in pediatric infection and immunity at Oxford.
“I think we’re getting close, and it’s definitely going to be before Christmas based on the progress,” Pollard told the BBC.
The strong immune response detected in older people is important because vaccines often don’t work as well in that cohort, Pollard said.
“The reason that we’re so delighted is that we’re seeing the immune responses look exactly the same, even in those who are over 70 years of age,” he said.
Those earlier findings are based on a Phase II trial of 560 people, including 240 over the age of 70. The results of the peer-reviewed study were published Thursday in the medical journal Lancet.
There are still many unknowns when it comes to the pandemic, but the early signs of success for two experimental COVID-19 vaccines make a few things clear.
Phase II vaccine trials provide important preliminary data but don’t prove whether they ultimately prevent people from getting sick. Oxford and AstraZeneca are waiting for the results of Phase III trials on thousands of people around the world to show whether their vaccine is safe and effective.
Two other drugmakers, Pfizer and Moderna, this week reported results from late-stage trials that showed their COVID-19 vaccines to be almost 95% effective.
Pollard said there was no competition among the various research teams, because several vaccines will be needed to bring the pandemic under control and allow life to return to normal.
Despite recent progress, Pollard said the world is still in the early stages of the effort to protect people against COVID-19. Even after vaccines are approved by regulators, drugmakers and public health officials still face the task of producing billions of doses and administering them to people around the world.
Pollard, an amateur mountaineer, compared the task to the work involved in climbing a mountain.
“I think we’re still at the bottom of that mountain in some ways,” he said. “We’ve done the route into the bottom of the mountain, the long trek to get to the start. Now we’ve got to get the data about the vaccines in front of regulators for them to scrutinize it and approve the first vaccines.
“And then we’ve got that huge effort to climb up to the top, where we’ve got a vast majority of those who are at risk vaccinated.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.