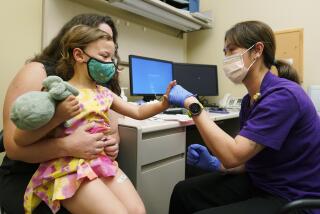
CDC recommends Pfizer-BioNTech vaccine for children as young as 12
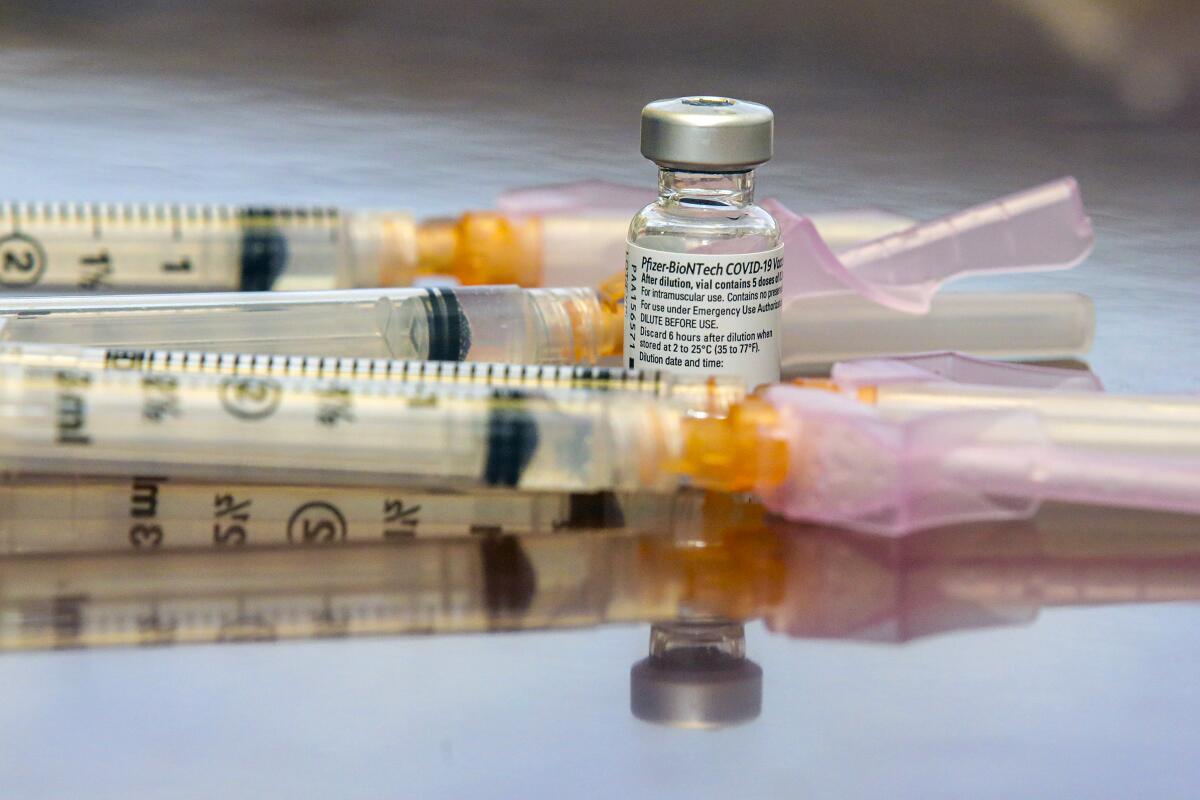
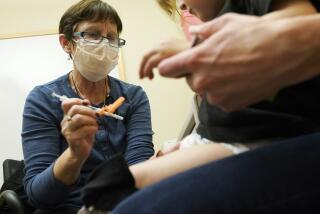
Children as young as 12 should receive the Pfizer-BioNTech coronavirus vaccine to protect them from COVID-19 and help end the pandemic, the U.S. Centers for Disease Control and Prevention recommended Wednesday.
The recommendation means that many middle school students and virtually all high school students will have the chance to be vaccinated before the next school year begins.
The vaccine is already available in the United States to everybody 16 and older. Making 12- to 15-year-olds eligible is an opportunity to vaccinate another 18 million people.
So far, 118 million people in the United States — or about 35% of the population — have been fully vaccinated. Experts say that figure has to at least double to reach herd immunity, the point at which the virus begins to die out, and that every group counts.
Though young people generally experience milder cases of COVID-19 than other groups, experts believe vaccinating adolescents will help protect their families and the broader community.
Vaccinating as many people as possible quickly also will drive down the likelihood that new variants will emerge.
“The higher the level of immunity in the population, the less spread of virus there will be — and, more importantly, less disease and death,” said Dr. Paul Spearman, the director of the Division of Infectious Diseases at Cincinnati Children’s Hospital.
As the vaccine becomes available to more adolescents, a key question will be how many parents will allow it. A survey conducted by the Kaiser Family Foundation suggested that, among parents of 12- to 15-year-olds, less than a third planned to have their children vaccinated as soon as possible.
About a quarter of the parents surveyed indicated they wanted to wait until more data became available, and almost as many said they didn’t plan on seeking vaccination for their children at all. Anyone younger than 18 who wants to be vaccinated must be accompanied by a parent or guardian.
The recommendation Wednesday to vaccinate children as young as 12 came from an independent panel of experts advising the CDC.
Calling it a step “closer to normalcy,” Dr. Rochelle Walensky, the CDC director, made the recommendation official policy later in the day, effectively endorsing the Food and Drug Administration’s recent decision to authorize the shot for emergency use in people ages 12 to 15.
In a recent clinical trial testing the Pfizer-BioNTech vaccine in that age group, 1,130 people received the vaccine and 1,129 got a placebo. No COVID-19 cases were reported among participants who were fully vaccinated, compared with 18 cases among those who received placebo shots.
The teenagers reported many of the same side effects as adults, including a sore arm, aches and chills, especially after the second dose. Based on the clinical data, the CDC panel said adolescents should receive the two full doses three weeks apart.
Vaccinating children is the critical next step in the U.S. campaign against COVID-19 — and one that public health officials worry could prove difficult.
Children are unlikely to become extremely ill or die from a coronavirus infection. Of the 582,000 U.S. deaths, 350 have been people between the ages of 5 and 17.
Infected adolescents, even those without symptoms, can still transmit the virus. Some teenagers who are out and about with friends might wear masks, stay outdoors and practice social distancing — but many will not. The CDC has reported that teenagers are twice as likely as younger children to test positive for the coronavirus.
Demand for COVID-19 vaccines has begun to drop across the United States, raising worries that the country will never reach herd immunity.
“One common challenge in communicating the need for vaccination is that many have rightly identified themselves as low risk for COVID-19 complications,” said Dr. C. Buddy Creech, the director of the Vanderbilt Vaccine Research Program. “This is true — teenagers, by and large, are able to navigate COVID-19 without a great deal of difficulty.”
Still, he said, “there are times when we face something that we can only solve together. The pandemic is a prime example of this. We vaccinate to protect ourselves and to protect one another.”
The rollout of the Pfizer-BioNTech vaccine has already been expanded to children 12 and older in Canada. The companies are seeking authorization for adolescents in the European Union as well.
Both Pfizer-BioNTech and Moderna have begun testing vaccines in children as young as 6 months to determine the right dosage for various age groups. Pfizer expects to report early results in late 2021.
In Britain, the AstraZeneca vaccine is being studied in children as young as 6, and in China, Sinovac has said its vaccine is safe for those as young as 3.
Staff writer Deborah Netburn contributed to this report.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.