Supreme Court’s antiabortion conservatives could restrict abortion pills sent by mail, even in blue states
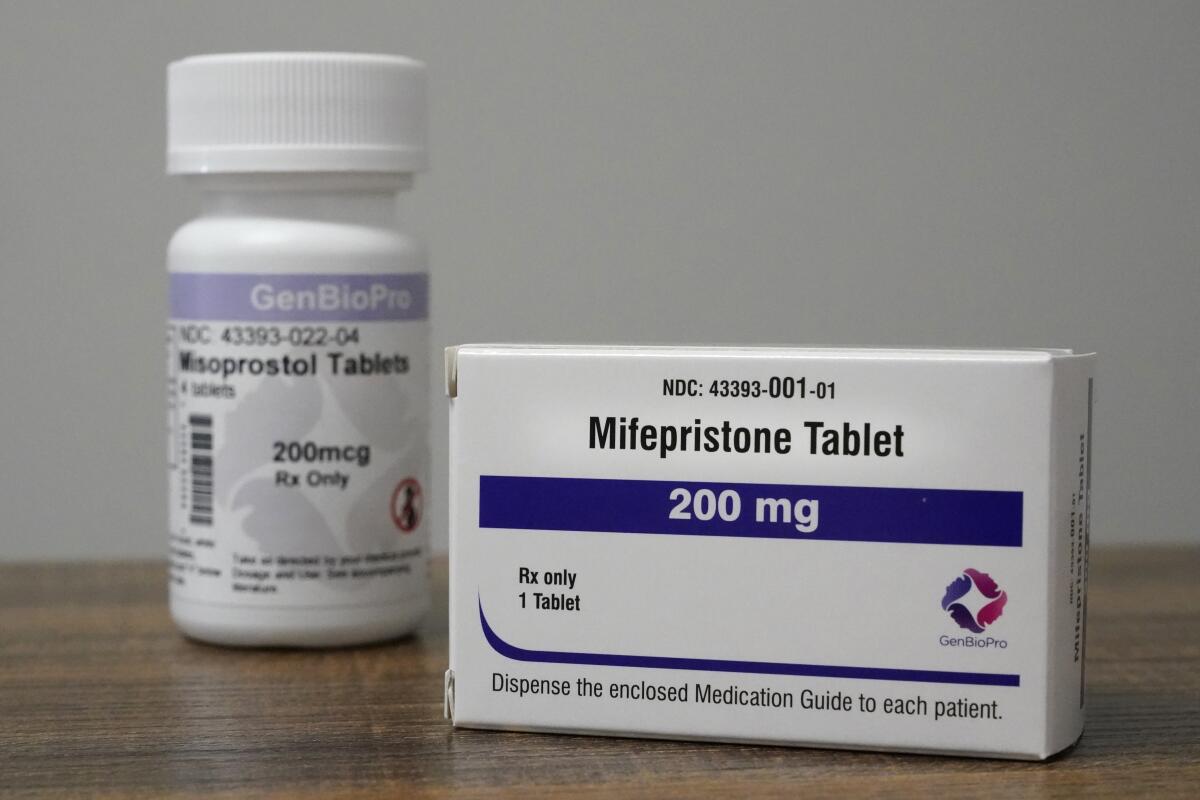
The Supreme Court’s antiabortion majority is set to consider whether to order a reversal in U.S. drug laws and restrict women from obtaining abortion medication at pharmacies or through the mail.
A ruling to restrict the most common method of abortion would limit the rights of women in California and other states where abortion remains legal.
“We may have thought we were protected because California is supportive of abortion, but this decision [on abortion pills] will be national in scope,” said Dr. Daniel Grossman, a professor of obstetrics at UC San Francisco.
The case is the most significant abortion question to come before the court since it overturned the constitutional right to abortion.
In that Dobbs ruling two years ago, the 5-4 majority sought to reassure those who live in states where abortion remains protected. Judges should not make the rules on abortion, they said, promising to return “that authority to the people and their elected representatives.”
But that pledge faces a major test. Conservative judges in Texas have shown themselves very willing to rewrite the rules for abortion medication and to impose their rules in blue states.
Whether they prevail now depends on the three conservative justices appointed by President Trump who were crucial to overturning Roe vs. Wade: Justices Neil M. Gorsuch, Brett M. Kavanaugh and Amy Coney Barrett. If all three join with Justices Clarence Thomas and Samuel A. Alito, they could roll back the use of abortion pills.
“It could eliminate telemedicine and reimpose the in-person dispensing requirement,” Grossman said. “It could also set a very bad precedent for the FDA.”
Conservative Supreme Court refused to second-guess the FDA’s approval of abortion pills as safe and effective.
Several years after the drug mifepristone had been legalized in much of Europe, the Food and Drug Administration in 2000 approved its use as a safe and effective means of ending an early pregnancy. Since 2016, the agency has liberalized its regulations to permit patients to consult a doctor through telemedicine and to obtain the pills without traveling to a clinic. It is now part of a two-drug combination accounting for more than half of U.S. abortions.
When taken in combination with misoprostol, the pills cause cramping and some bleeding. It can sometimes require a doctor’s intervention to complete the abortion, but the FDA says serious complications are “exceedingly rare,” noting that more than 5 million women in the U.S. have used the medication since 2000.
More than a dozen major medical groups, led by the American College of Obstetricians & Gynecologists and the American Medical Assn., said in friend-of-the court briefs that two decades of studies have shown the drugs are safe.
“When used in medication abortion, major adverse events — significant infection, excessive blood loss or hospitalization — occur in less than .32% of patients,” they wrote.
On Tuesday, however, the court will hear a broad attack on the FDA from attorney Erin M. Hawley, the wife of Republican Missouri Sen. Josh Hawley and a former law clerk to Chief Justice John G. Roberts. She says the court should not turn “a blind eye to FDA’s patently unreasonable actions here, which jeopardize women’s health throughout the nation.”
Hawley is not representing women who say they were injured by the drugs or doctors who prescribe the medication. Instead, she is representing a group of doctors who oppose abortion on religious and moral grounds.
She argues they have legal standing to sue the FDA because some of the group’s members work in emergency rooms, and they could be forced to treat patients who took abortion pills and went to a hospital because of bleeding or other complications.
“When faced with these emergencies, [the doctors] have no choice but to provide immediate treatment, even though this kind of participation in an elective abortion harms their consciences and injures them in other ways,” Hawley wrote in her brief to the court.
Two studies cited by a judge in a ruling restricting the abortion medication mifepristone were retracted. But they’re still being cited in an appeal to the Supreme Court.
Shortly after the Supreme Court repealed the right to abortion in the Dobbs case, Hawley filed a lawsuit in Amarillo, Texas, seeking the repeal of the FDA’s approval of mifepristone.
The location was no accident. Her suit would come before U.S. District Judge Matthew Kacsmaryk, a Trump appointee who has been an outspoken foe of same-sex marriage and LGBTQ+ rights. Kacsmaryk had served as deputy general counsel for First Liberty Institute, a Christian conservative nonprofit that works on religious freedom cases, and had criticized the Supreme Court’s landmark 1973 Roe vs. Wade decision before its reversal.
In past decades, conservatives often railed against “judicial activism” and judges who “legislate from the bench.” In recent years, however, conservative activists have gone before judges in red states seeking to win major changes in the law that they could not win from Congress or the White House.
As predicted, Kacsmaryk handed down a broad ruling against what he called “chemical abortion” and ordered the FDA “to suspend” its approval of the drugs. In a ruling using language common to antiabortion groups, Kacsmaryk said the studies submitted by the FDA did not convince him mifepristone was safe.
The government rushed to appeal last April, and the U.S. Court of Appeals for the 5th Circuit agreed to limit Kacsmaryk’s ruling. By a 2-1 vote, the appeals court said it was too late to unravel the approval of the drug in 2000, but not too late to overturn the FDA’s regulations that since 2016 have made it easier for women to obtain the pills.
Biden administration Solicitor Gen. Elizabeth B. Prelogar called the case a first.
She said it “marks the first time any court has restricted access to an FDA-approved drug by second-guessing FDA’s expert judgment about the conditions required to assure that drug’s safe use.”
She urged the Supreme Court to put the lower court rulings on hold, and the justices did so over dissents by Thomas and Alito. A few months later, the court agreed to rule in the case of FDA vs. Alliance for Hippocratic Medicine.
Abortion-rights advocates are troubled the case has gotten so far.
Texas’ controversial immigration law is on hold again after court moves that confounded the Biden administration and spurred outrage from Mexico’s government.
“This makes a mockery of our legal system,” said Julia Kaye, an attorney for the American Civil Liberties Union. “This is a group of antiabortion extremists going before a hand-picked judge and using junk science and sham claims about women’s health to serve their true goal of making abortion inaccessible.”
The solicitor general, representing the FDA, will urge the court to end the case quietly by throwing out the lawsuit.
Usually, the court has said plaintiffs have standing to sue over a law or regulation only if they are or will be personally injured by it.
The antiabortion doctors who sued “do not prescribe mifepristone, and FDA’s actions allowing other providers to prescribe the drug do not require them to do or refrain from doing anything,” Prelogar wrote in her brief to the court. They “have not identified even a single doctor among their thousands of members who has ever been required to perform an abortion in the decades mifepristone has been on the market.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.